Introduction – Rule 71(3):
After the European Patent Office (‘EPO’) issues a Rule 71(3) notification indicating its intention to grant a European patent, the applicant must respond within a non-extendable period of four months from the notification date. Failure to do so will result in the application being deemed withdrawn.
To proceed, the applicant must:
- Approve the text as provided by the EPO, and verify the bibliographic data;
- Pay the grant fee, which includes the publication fee and any additional claims fees; and
- File translations of the claims into the other two official languages of the EPO (French and German, if the application is in English).
Alternatively, if the applicant wishes to amend or correct the text, they can submit reasoned amendments. If the EPO accepts these amendments, the application will proceed to grant.
Once the text is agreed upon, all fees are paid, and translations are filed, the patent will be granted, and the mention of the grant will be published in the European Patent Bulletin (‘date of grant’).
If required, a divisional application must be filed before the date of grant i.e., while the application is still pending.
Any renewal fee due before the date of grant must also be paid to the EPO. The obligation to pay renewal fees to the EPO ends after the renewal fee for the year of the grant publication is paid, and renewals thereafter are paid to the respective National Patent Offices.
Validations:
The European Patent Convention (EPC) provides a centralized grant procedure, but after grant, the European patent essentially becomes a bundle of national patents.
After a European patent is granted, it must be validated in each EPC member state where the applicant seeks protection. This process must be completed within three months from the date of grant.
EPC Member States:
The European Patent Convention (EPC) includes a broad range of member states across Europe and beyond, where a European patent can be validated. These countries have different requirements, such as translations, fees, and the need for local representation.
The 39 EPC Member states are:
Albania, Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Croatia, Hungary, Iceland, Ireland, Italy, Liechtenstein, Lithuania, Luxembourg, Latvia, Malta, Monaco, Montenegro, Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Türkiye, and the United Kingdom.
Validation States:
Since 2010, the EPO has signed validation agreements with seven non-member states, five of which have entered into force:
Cambodia, Georgia, Laos (with validation agreement signed on 13.5.2024 and entering into force on 1.4.2025), Morocco, Republic of Moldova and Tunisia.
An agreement Costa Rica was signed on 13.12.2024, and is not yet in force.
Extension States:
Between 1993 and 2009, the EPO signed extension agreements with ten non-member states, however, the only extension agreement in currently in force is with Bosnia and Herzegovina.
Translations under the London Agreement:
The claims of a European patent will always be available in all three official languages of the EPO: English, French, and German, as part of the European patent grant requirements.
The London Agreement aims to reduce further translation costs for European patents by waiving or minimizing translation requirements when validating a granted European patent in the EPC Member states.
There are 22 EPC member states that are parties to the London Agreement, which provides that:
The following states have an official language in common with one of the official languages of the EPO and do not require further translations of a European patent granted in an official language of the EPO:
Belgium, France, Germany, Ireland, Liechtenstein, Luxembourg, Monaco, Switzerland, and the United Kingdom.
The following states do not have an official language in common with one of the official languages of the EPO, and require the claims of a European patent granted in an official language of the EPO to be translated into one of their own national languages or into the prescribed language nominated by these states, which is in English:
Albania, Croatia, Denmark, Finland, Hungary, Iceland, Netherlands, Norway, Sweden.
The following states do not have an official language in common with one of the official languages of the EPO, and require the claims of a European patent granted in an official language of the EPO to be translated into one of their own national languages and no other prescribed language:
North Macedonia, Latvia, Lithuania, Slovenia.
Unitary Patent:
Instead of validating a European patent separately in multiple countries, applicants can request unitary effect, allowing the patent to take effect in all participating EU member states without the need for individual national validations. This process can potentially reduce both costs and administrative burden. Aside from territorial coverage, there are of course other factors to consider, as previously discussed [1]-[4].
To obtain a Unitary patent, i.e., unitary effect from a European patent, the applicant must file a written request to the EPO within one month of the date of grant.
During a transitional period of the Unitary Patent System (six years, which may be extended up to a maximum of 12 years), a translation of the European patent specification must be filed alongside the request for unitary effect, according to the following:
If the language of the proceedings before the EPO was French or German, the translation must be filed into English; or if the language of the proceedings was English, the translation must be filed into any other official language of an EU member state of the Unitary patent system.
Once the transitional period expires, no further translation will be required to obtain a Unitary patent.
A translation of the claims into the EPO’s other two official languages will of course already have been filed at the end of the grant procedure.
Therefore, if one of these languages is chosen for the translation, only the description needs to be additionally translated when filing the request for unitary effect. The translation of the claims could potentially be reused.
The EPO will then examine the request and confirm the unitary effect if all requirements are met, and a Unitary patent will be effected.
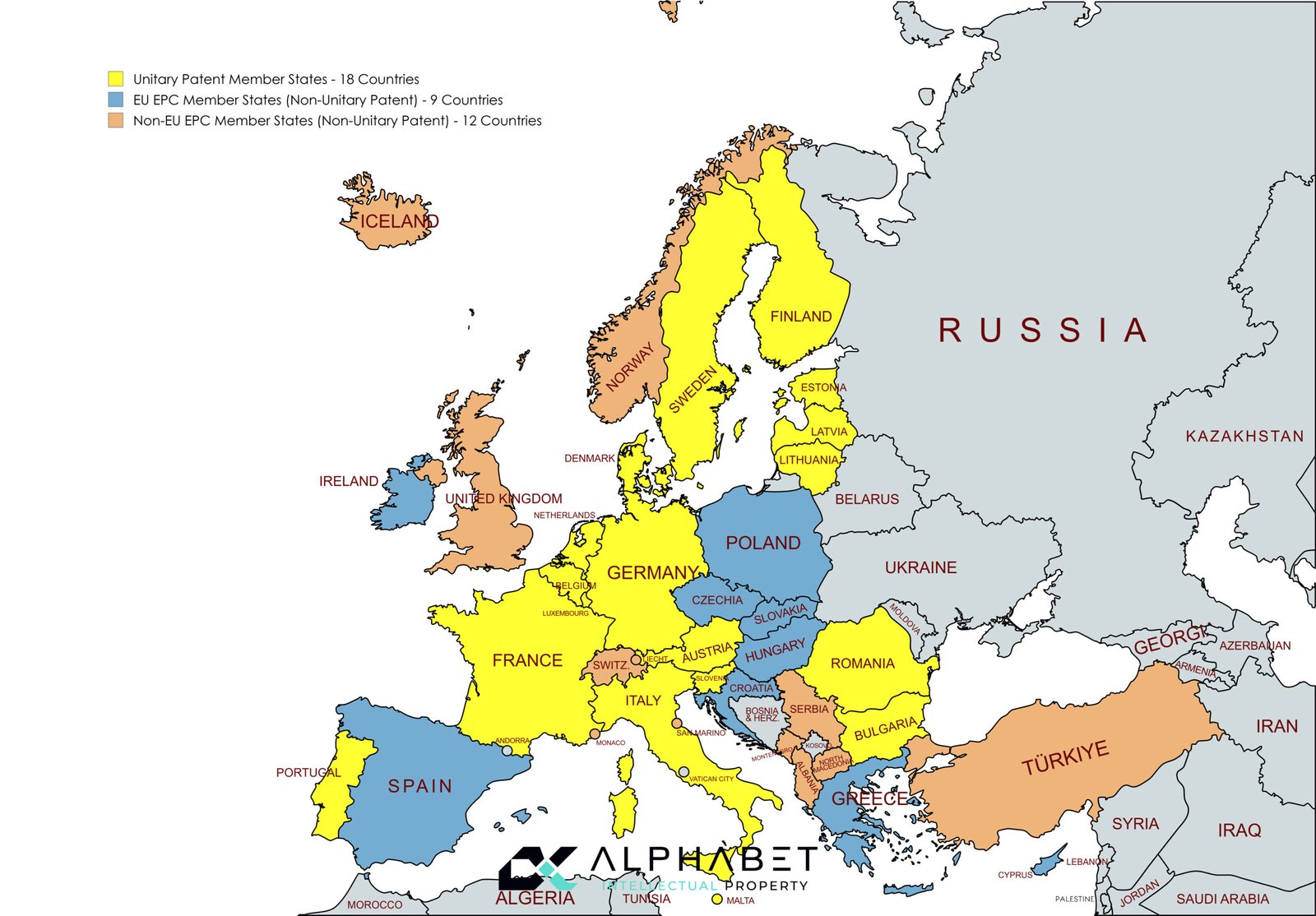
European Patent vs Unitary Patent Coverage:
There are 27 EU-member states eligible to join the Unitary patent system. The 18 states in enhanced cooperation which already ratified the Agreements and participate in the Unitary Patent are (Yellow):
Austria, Belgium, Bulgaria, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Portugal, Romania, Slovenia, Sweden.
The 7 states in enhanced cooperation which have not ratified the Agreement are:
Cyprus, Czech Republic, Greece, Hungary, Ireland, Poland and Slovakia.
The two states that have opted out of the Unitary Patent System are:
Croatia, and Spain.
Non-Unitary Patent EPC Member States:
There are a total of 21 EPC member states for which patent protection cannot be obtained via unitary effect but which can be obtained via validating a European patent.
9 EU and EPC Member states are (Blue):
Croatia, Cyprus, Czech Republic, Greece, Hungary, Ireland, Poland and Slovakia, and Spain.
12 Non-EU EPC Member states are (Deep orange):
Albania, Iceland, Liechtenstein, Monaco, Montenegro, North Macedonia, Norway, San Marino, Serbia, Switzerland, Türkiye, and the United Kingdom.
As previously mentioned, there are in addition via a European patent, the Validation states are:
Cambodia, Georgia, Morocco, Republic of Moldova and Tunisia;
and Extension state is:
Bosnia and Herzegovina.
Conclusion:
In summary, the European Patent System and the Unitary Patent System operate in parallel once a European patent application is granted, and the decision between the two or both largely depends in part on the desired territorial coverage.
While the Unitary patent provides streamlined protection across 18 EU member states that have ratified the Unitary Patent Agreement, applicants should be aware that the European patent still requires validation in at least 21 non-participating countries.
Even if an applicant opts for a Unitary patent, the formalities for granting the European patent must be completed in full first, including the payment of the grant fee and translations of the claims into the other two official languages of the EPO; and for non-EU member states or countries not covered by the Unitary patent, separate national validations are still required.
Therefore, understanding the territorial scope of a European patent as compared with a Unitary patent is crucial for deciding the most effective strategy, and it is important to recognize that even after the Unitary patent is granted, there are still additional steps to take in countries not covered by opting for a Unitary patent.
As of now, 21 EPC member states fall outside the Unitary Patent system, which underscores the need for a clear strategy hence conventional validation requirements.
U is for Understanding the territorial scope of a Unitary Patent, which is crucial for deciding whether to pursue a Unitary Patent and/or the traditional European Patent route.
References:
[1] Unitary Patent: Territorial Scope of the Unitary Patent System – https://alphabetip.co.uk/the-patent-alphabetical/unitary-patent-territorial-scope-of-the-unitary-patent-system/
[2] Unitary Patent: Unitary Effect & Opt-out – https://alphabetip.co.uk/the-patent-alphabetical/unitary-patent-unitary-effect-and-opt-out/
[3] Unitary Patent: Cost Savings Opportunity – https://alphabetip.co.uk/the-patent-alphabetical/unitary-patent-cost-savings-opportunity/
[4] Unitary Patent: Romania 18th Member State to ratify UPCA – https://alphabetip.co.uk/the-patent-alphabetical/upc-unitary-patent-romania/
Keep up with our Unitary Patent System series and other interesting Intellectual Property content by following our LinkedIn company page – Alphabet Intellectual Property.
To find out more about patents and intellectual property, or if you have any questions or would like to get in touch, please feel free to email us at info@alphabetip.co.uk